
The Scalpel and the Algorithm: How GPT Vision is Revolutionizing Oncology Diagnostics
The Dawn of a New Era in Medical Imaging and Diagnosis
The convergence of artificial intelligence and medicine has transitioned from a futuristic concept to a clinical reality, heralding one of the most significant transformations in modern healthcare. At the forefront of this revolution are advanced multimodal AI systems, particularly Generative Pre-trained Transformer (GPT) models equipped with vision capabilities. While earlier iterations of AI focused on text-based data, the latest GPT Vision News reveals a profound leap: the ability to interpret and reason about complex visual information. This has unlocked unprecedented potential in specialties that are heavily reliant on medical imaging, with oncology standing out as a primary beneficiary. In the intricate world of cancer diagnosis, where the microscopic analysis of tissue can mean the difference between life and death, GPT Vision is emerging not as a replacement for human experts, but as a powerful computational co-pilot. This article delves into the technical architecture, clinical applications, and ethical considerations of using GPT Vision to analyze histopathology slides, enhance diagnostic accuracy, and personalize cancer treatment in ways previously thought impossible. The latest developments in GPT in Healthcare News are not just incremental; they represent a paradigm shift in our fight against cancer.
Section 1: The Architectural Leap: From Text to Tissue with GPT Vision
The ability of an AI to “read” a pathology slide is not magic; it is the result of sophisticated architectural innovations that bridge the gap between pixels and clinical insights. Understanding this foundation is key to appreciating both its capabilities and its limitations.
Beyond Text: Understanding Multimodal AI
The journey from text-only models like GPT-3.5 to the multimodal powerhouses of today, such as GPT-4 with Vision, marks a critical evolution in AI. The core challenge lies in creating a unified model that can seamlessly process and correlate information from disparate sources—text, images, and structured data. The latest GPT Architecture News highlights the integration of two key components: a Vision Transformer (ViT) and a Large Language Model (LLM). The ViT acts as the model’s “eyes.” It ingests a high-resolution image, such as a digitized histopathology slide, and breaks it down into a grid of smaller patches. Each patch is then converted into a numerical vector, or embedding, that captures its visual features. These image embeddings are then passed to the LLM, which processes them alongside text-based prompts. This fusion allows the model to perform complex, cross-modal reasoning, answering questions about an image in natural language.
How GPT Vision “Sees” a Histopathology Slide
When a pathologist examines a tissue sample, they are performing a highly trained act of pattern recognition. GPT Vision emulates and augments this process at a massive scale. A whole-slide image (WSI), which can be gigapixels in size, is fed into the system. The ViT component scans the entire slide, encoding its visual data. This process is informed by extensive pre-training on vast datasets of medical images, a key topic in GPT Training Techniques News. The model learns to identify cellular structures, tissue architectures, mitotic figures (cells undergoing division), and signs of necrosis or invasion. When a clinician provides a prompt—for example, “Identify all regions with high-grade pleomorphism and provide a count of mitotic figures per high-power field”—the LLM component uses the encoded visual data to generate a precise, context-aware response. This might include highlighting specific regions on the slide image and providing a text-based summary, effectively translating visual evidence into actionable clinical data. This powerful combination of visual perception and linguistic reasoning is central to the latest GPT Multimodal News.
Section 2: Clinical Applications in Oncology: A Paradigm Shift in Decision-Making
The theoretical power of GPT Vision translates into tangible, practice-altering applications across the entire oncology workflow, from initial diagnosis to personalized treatment planning. The impact is not merely about efficiency; it is about fundamentally enhancing the quality and precision of care.
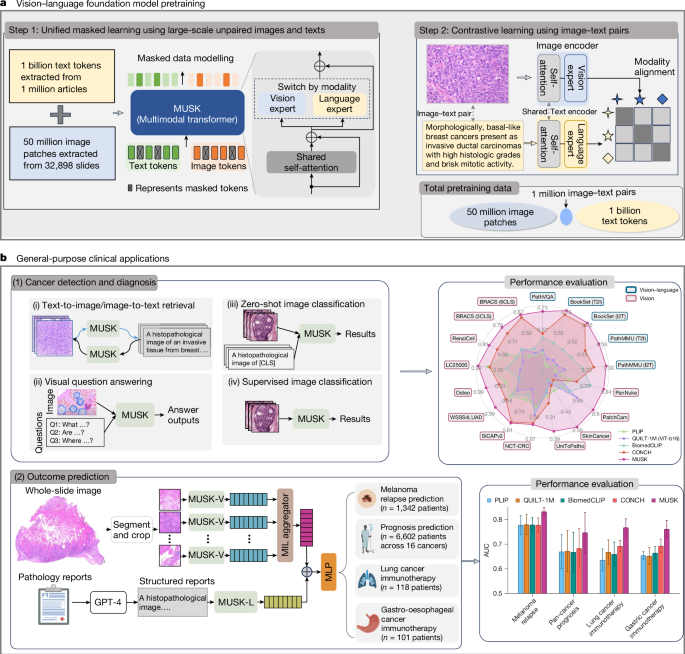
Enhancing Diagnostic Accuracy and Speed
A pathologist’s workload is immense, often involving the review of hundreds of slides per day. This can lead to fatigue and variability in interpretation. GPT Vision tools can serve as a tireless “second reader.” In a real-world scenario, an AI assistant could pre-screen a batch of biopsies, flagging slides with a high probability of malignancy and highlighting specific regions of interest. This allows the human expert to focus their attention where it is most needed, reducing turnaround times and minimizing the risk of missed diagnoses. Recent studies, a hot topic in GPT Benchmark News, have shown that AI-augmented workflows can dramatically improve diagnostic accuracy. In some specialized tasks, such as grading tumors or identifying rare cancer subtypes, AI assistance has been shown to boost the consensus accuracy among pathologists from below 50% to over 85%, demonstrating its power to standardize and elevate the quality of care.
Precision Medicine and Genomic Correlation
One of the most groundbreaking applications discussed in GPT Research News is the ability to predict genetic mutations directly from a tissue slide’s appearance—a field known as “histogenomics.” Certain genetic mutations, like EGFR in lung cancer or BRAF in melanoma, cause subtle but distinct changes in cellular morphology (the “phenotype”). By training on datasets that pair slide images with their corresponding genomic sequencing data, GPT Custom Models can learn to identify these visual signatures. A pathologist, aided by AI, could look at a slide and receive a probability score for the presence of a key mutation. This could enable clinicians to initiate targeted therapy much faster, without waiting days or weeks for next-generation sequencing results, marking a significant step forward for personalized medicine.
Automating Clinical Tool Execution and Guideline Adherence
Modern oncology is governed by complex, constantly evolving clinical guidelines from organizations like the NCCN or ASCO. GPT Vision models are evolving into GPT Agents that can not only analyze data but also execute clinical protocols. For example, a clinician could upload a patient’s pathology report and slide image and prompt the model: “Based on the attached data, stage this colorectal adenocarcinoma according to the AJCC 8th edition guidelines, cite the specific criteria used for the T, N, and M stages, and list the recommended adjuvant therapies.” The model can parse the text, analyze the image for depth of invasion, and cross-reference the information against its knowledge base of clinical guidelines. Recent demonstrations show these models can execute such tasks with over 85% accuracy and cite the correct guidelines in over 75% of cases, acting as a real-time decision support tool that ensures compliance and best practices.
Section 3: Technical Hurdles and Ethical Imperatives on the Road to Deployment
Despite the immense promise, the path to widespread clinical adoption of GPT Vision is fraught with significant technical, ethical, and logistical challenges. Addressing these issues transparently is crucial for building trust and ensuring patient safety.
The “Black Box” Problem and Interpretability
One of the most significant barriers to adoption is the “black box” nature of deep learning models. For a clinician to trust an AI’s recommendation, they need to understand *why* it was made. The latest GPT Ethics News emphasizes the need for explainability. If a model flags a region as malignant, it must be able to show its work. Techniques like attention maps, which overlay a heatmap on the original image to show which pixels the model focused on, are a step in the right direction. However, achieving true clinical-grade interpretability—where the model can articulate its reasoning in a way that aligns with a pathologist’s own diagnostic process—remains an active area of research covered in GPT Safety News.
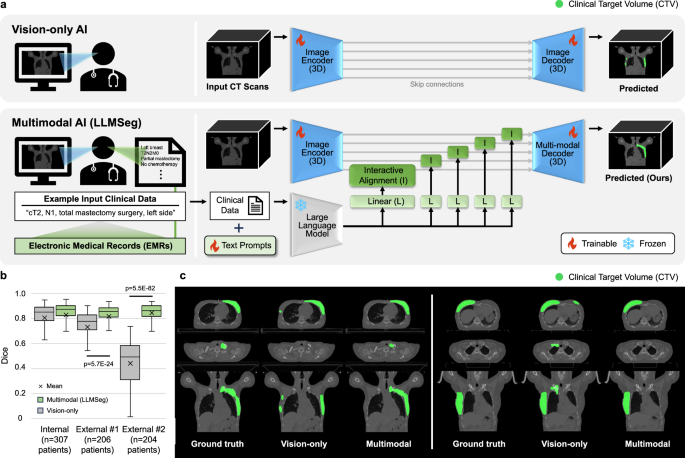
Data Scarcity, Privacy, and Bias
High-quality, well-annotated medical datasets are the lifeblood of AI development, but they are also difficult and expensive to create. Furthermore, patient data is protected by stringent regulations like HIPAA and GDPR, a central theme in GPT Privacy News. Training models requires vast amounts of data, raising concerns about privacy. Innovations like federated learning, where the model is trained locally at different hospitals without the data ever leaving the premises, offer a potential solution. An even greater concern is bias. If a model is trained primarily on data from one demographic, it may perform poorly on others, exacerbating existing health disparities. Rigorous auditing for fairness and ensuring diverse training data are non-negotiable prerequisites for deployment, a key focus of GPT Bias & Fairness News and emerging GPT Regulation News.
Integration and Workflow Disruption
A brilliant algorithm is useless if it cannot be integrated into a clinician’s daily workflow. The practical challenges of deployment are immense. This involves creating robust GPT APIs that can connect with existing hospital IT infrastructure, such as Electronic Health Records (EHRs) and Picture Archiving and Communication Systems (PACS). The user interface of these GPT Tools must be intuitive and seamless, augmenting rather than disrupting the pathologist’s process. The latest GPT Deployment News underscores that success is as much about human-computer interaction and systems engineering as it is about the AI model itself.
Section 4: Best Practices for Implementation and the Future Horizon
Navigating the complexities of clinical AI requires a strategic approach focused on collaboration, validation, and a clear vision for the future. As technology accelerates, particularly with the anticipation of GPT-5 News, establishing a robust framework for implementation is paramount.
Recommendations for Clinical Adoption
For healthcare organizations looking to leverage these technologies, a phased and cautious approach is essential.
- Start with Augmentation, Not Automation: Initially, AI should be positioned as a decision support tool or a “second reader” to assist human experts. Full automation in diagnostics is a distant and perhaps undesirable goal. The focus should be on human-AI collaboration.
- Rigorous and Localized Validation: A model that performs well in a lab may not perform well in a specific hospital’s clinical environment due to differences in patient populations, lab equipment, or slide preparation techniques. It is critical to validate any AI tool on local data before clinical use.
- Establish Continuous Monitoring: AI models are not static. Their performance can drift over time as clinical practices or patient demographics change. Implementing a system for continuous monitoring, feedback, and retraining is crucial for long-term safety and efficacy, a key aspect of GPT Optimization News.
The Road Ahead: GPT-5 and Beyond
The future of AI in oncology is holistic. The next generation of models, hinted at in early GPT Future News, will likely move beyond just histopathology. We can envision a single, unified AI that integrates a patient’s entire medical record: their genomic data, radiology scans (CT, MRI), pathology slides, and clinical notes. This model could provide a comprehensive, longitudinal view of a patient’s cancer, predicting treatment response, and identifying early signs of recurrence. Furthermore, developments in GPT Edge News and GPT Efficiency News point towards smaller, specialized models that could run in real-time on devices in the operating room, providing surgeons with live feedback on tumor margins. The pace of innovation in the GPT Ecosystem is staggering, promising a future where AI is an indispensable, integrated partner in delivering truly personalized cancer care.
Conclusion: A New Partnership in Patient Care
The integration of GPT Vision into oncology represents a watershed moment for medicine. By translating complex visual data from pathology slides into actionable clinical insights, these AI models are poised to significantly enhance diagnostic accuracy, accelerate workflows, and usher in an era of visually-guided precision medicine. The journey is not without its challenges; ethical considerations around bias, privacy, and interpretability must be diligently addressed, and seamless clinical integration remains a significant hurdle. However, the potential benefits are too profound to ignore. As the technology matures, the collaboration between AI developers and medical professionals will be the cornerstone of progress. The latest OpenAI GPT News continues to push the boundaries of what’s possible, solidifying a future where the algorithm and the scalpel work in concert to improve and save lives.



